- Ionization energy is defined as the energy required to remove an electron from a neutral atom.
- Electronegativity is a measure of the tendency of an atom to attract or gain an electron. Nonmetals are usually said to be electronegative; metals, electropositive.
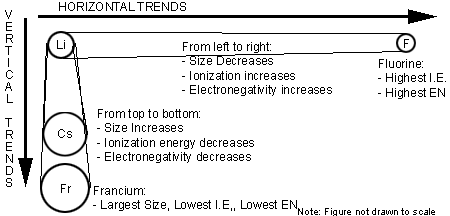
- Atomic size for the reactive elements is based on atomic radii that are based on bonding distances in compounds.
Based on the observed trends, francium and cesium (on the bottom left), are the most metallic elements (i.e. the most likely to lose an electron) while fluorine (on the upper right) is the most nonmetallic (i.e. the most likely to gain an electron).
The above periodic trends are caused by the interactions of three factors: nuclear charge (the number of protons in the nucleus), the electron shell(s), and the shielding (the effect of the electrons between the outer electrons and the nucleus). These effects are summarized in Table 1 below. Similar arguments can be used to explain the trends in ionization energy and electronegativity.
Table 1. Factors affecting horizontal and vertical trends in atomic size:
| Horizontal Behavior | Vertical Behavior | |||
| Factors: | Change | Effect on Size | Change | Effect on Size |
| Atomic number: number of protons | increases | decreases | increases | decreases |
| Electron shell(s) | stays same | no effect | shell added | increases |
| Shielding (intervening electrons) | stays same | no effect | increases | increases |
Net Effect:
| decreases* | increases* | ||
Another helpful bit of information is a list of the common nonmetals in decreasing order of electronegativity - F, O, Cl, N, Br, I, S, C……H - (pronounced fossil n brisk to help memorize).
This, along with geometric considerations, will help to determine the polarity (another lesson) of small covalent compounds.
No comments:
Post a Comment